Sbírka 69+ Atom Quantum Numbers Zdarma
Sbírka 69+ Atom Quantum Numbers Zdarma. To completely describe an electron in an atom, four quantum numbers are needed: The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). 17.01.2021 · in atoms, there are a total of four quantum numbers: The first quantum number describes the electron shell, or energy level, of an atom.
Tady Internal Electric Field Strength Of Muonic Atom For Each Principal Download Scientific Diagram
Each electron in an atom is described by four different quantum numbers. In other words, it refers to … Describes the shape of the orbital. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s).There are four quantum numbers for atoms:
The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus. Each electron in an atom is described by four different quantum numbers. To completely describe an electron in an atom, four quantum numbers are needed: The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus.

N = 1, 2, 3, …, 8. #n = 1, 2, 3,. Quantum numbers can be used to describe the quantum state of an electron. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). N = 1, 2, 3, …, 8. Each electron in an atom is described by four different quantum numbers. 17.01.2021 · in atoms, there are a total of four quantum numbers: Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). There are four quantum numbers for atoms: To completely describe an electron in an atom, four quantum numbers are needed: Describes the shape of the orbital... Quantum numbers can be used to describe the quantum state of an electron.
The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s)... Each electron in an atom is described by four different quantum numbers. #0 harr s, 1 harr p, 2 harr d, 3 harr f,. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). #n = 1, 2, 3,. In other words, it refers to … The value of n ranges from 1 to the shell containing the outermost electron of that atom. The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. The first quantum number describes the electron shell, or energy level, of an atom. Quantum numbers can be used to describe the quantum state of an electron.. Each electron in an atom is described by four different quantum numbers.

The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital... N = 1, 2, 3, …, 8. To completely describe an electron in an atom, four quantum numbers are needed: #0 harr s, 1 harr p, 2 harr d, 3 harr f,. The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital.

Each electron in an atom is described by four different quantum numbers. The first quantum number describes the electron shell, or energy level, of an atom.

Quantum numbers can be used to describe the quantum state of an electron... In other words, it refers to … There are four quantum numbers for atoms:

#n = 1, 2, 3,... 17.01.2021 · in atoms, there are a total of four quantum numbers: In other words, it refers to …. Describes the shape of the orbital.

17.01.2021 · in atoms, there are a total of four quantum numbers: There are four quantum numbers for atoms: Quantum numbers can be used to describe the quantum state of an electron. #0 harr s, 1 harr p, 2 harr d, 3 harr f,. Describes the shape of the orbital.

Each electron in an atom is described by four different quantum numbers.. The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. There are four quantum numbers for atoms: #n = 1, 2, 3,. Each electron in an atom is described by four different quantum numbers.. The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital.

Describes the shape of the orbital. Describes the shape of the orbital. N = 1, 2, 3, …, 8. #n = 1, 2, 3,. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). To completely describe an electron in an atom, four quantum numbers are needed: Quantum numbers can be used to describe the quantum state of an electron. In other words, it refers to … #0 harr s, 1 harr p, 2 harr d, 3 harr f,.

The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus... 17.01.2021 · in atoms, there are a total of four quantum numbers: Quantum numbers can be used to describe the quantum state of an electron. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). #l = 0, 1, 2,. #n = 1, 2, 3,. To completely describe an electron in an atom, four quantum numbers are needed: The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. Describes the shape of the orbital. There are four quantum numbers for atoms:.. #l = 0, 1, 2,.

There are four quantum numbers for atoms: The first quantum number describes the electron shell, or energy level, of an atom. The value of n ranges from 1 to the shell containing the outermost electron of that atom. Describes the shape of the orbital. #n = 1, 2, 3,. N = 1, 2, 3, …, 8. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus. #0 harr s, 1 harr p, 2 harr d, 3 harr f,.

In other words, it refers to …. In other words, it refers to … 17.01.2021 · in atoms, there are a total of four quantum numbers: The first quantum number describes the electron shell, or energy level, of an atom. To completely describe an electron in an atom, four quantum numbers are needed: Quantum numbers can be used to describe the quantum state of an electron. Describes the shape of the orbital. The value of n ranges from 1 to the shell containing the outermost electron of that atom.. Describes the shape of the orbital.

The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s).. The first quantum number describes the electron shell, or energy level, of an atom. To completely describe an electron in an atom, four quantum numbers are needed: #n = 1, 2, 3,. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. #l = 0, 1, 2,.. #n = 1, 2, 3,.

17.01.2021 · in atoms, there are a total of four quantum numbers: Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). To completely describe an electron in an atom, four quantum numbers are needed:. #0 harr s, 1 harr p, 2 harr d, 3 harr f,.

Quantum numbers can be used to describe the quantum state of an electron.. #l = 0, 1, 2,. The value of n ranges from 1 to the shell containing the outermost electron of that atom.

The first quantum number describes the electron shell, or energy level, of an atom. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). The value of n ranges from 1 to the shell containing the outermost electron of that atom. #0 harr s, 1 harr p, 2 harr d, 3 harr f,. To completely describe an electron in an atom, four quantum numbers are needed: Each electron in an atom is described by four different quantum numbers... In other words, it refers to …

#0 harr s, 1 harr p, 2 harr d, 3 harr f,. In other words, it refers to … Quantum numbers can be used to describe the quantum state of an electron. Each electron in an atom is described by four different quantum numbers. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). The first quantum number describes the electron shell, or energy level, of an atom. #l = 0, 1, 2,. The value of n ranges from 1 to the shell containing the outermost electron of that atom. N = 1, 2, 3, …, 8. The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus.. #0 harr s, 1 harr p, 2 harr d, 3 harr f,.

To completely describe an electron in an atom, four quantum numbers are needed: #n = 1, 2, 3,. #0 harr s, 1 harr p, 2 harr d, 3 harr f,. Quantum numbers can be used to describe the quantum state of an electron. To completely describe an electron in an atom, four quantum numbers are needed: Describes the shape of the orbital. The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). N = 1, 2, 3, …, 8.

To completely describe an electron in an atom, four quantum numbers are needed: The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. N = 1, 2, 3, …, 8. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). The first quantum number describes the electron shell, or energy level, of an atom. In other words, it refers to … To completely describe an electron in an atom, four quantum numbers are needed: Describes the shape of the orbital. #n = 1, 2, 3,. Each electron in an atom is described by four different quantum numbers. There are four quantum numbers for atoms: 17.01.2021 · in atoms, there are a total of four quantum numbers:
Quantum numbers can be used to describe the quantum state of an electron. The value of n ranges from 1 to the shell containing the outermost electron of that atom. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus. 17.01.2021 · in atoms, there are a total of four quantum numbers: #n = 1, 2, 3,. N = 1, 2, 3, …, 8. To completely describe an electron in an atom, four quantum numbers are needed: The first quantum number describes the electron shell, or energy level, of an atom. Each electron in an atom is described by four different quantum numbers. Quantum numbers can be used to describe the quantum state of an electron. Describes the shape of the orbital.. Quantum numbers can be used to describe the quantum state of an electron.

To completely describe an electron in an atom, four quantum numbers are needed: .. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s).

Each electron in an atom is described by four different quantum numbers... Each electron in an atom is described by four different quantum numbers. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). There are four quantum numbers for atoms: #0 harr s, 1 harr p, 2 harr d, 3 harr f,. To completely describe an electron in an atom, four quantum numbers are needed: Describes the shape of the orbital. 17.01.2021 · in atoms, there are a total of four quantum numbers: The value of n ranges from 1 to the shell containing the outermost electron of that atom... The value of n ranges from 1 to the shell containing the outermost electron of that atom.

The value of n ranges from 1 to the shell containing the outermost electron of that atom. N = 1, 2, 3, …, 8. #n = 1, 2, 3,. There are four quantum numbers for atoms: To completely describe an electron in an atom, four quantum numbers are needed: In other words, it refers to … The value of n ranges from 1 to the shell containing the outermost electron of that atom. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus. Quantum numbers can be used to describe the quantum state of an electron. Describes the shape of the orbital.

The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s).. Quantum numbers can be used to describe the quantum state of an electron. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). To completely describe an electron in an atom, four quantum numbers are needed: Describes the shape of the orbital. The value of n ranges from 1 to the shell containing the outermost electron of that atom... The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus.

#0 harr s, 1 harr p, 2 harr d, 3 harr f,.. Quantum numbers can be used to describe the quantum state of an electron. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). To completely describe an electron in an atom, four quantum numbers are needed: #n = 1, 2, 3,. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). 17.01.2021 · in atoms, there are a total of four quantum numbers: In other words, it refers to … N = 1, 2, 3, …, 8. The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital.

Quantum numbers can be used to describe the quantum state of an electron... 17.01.2021 · in atoms, there are a total of four quantum numbers:

Quantum numbers can be used to describe the quantum state of an electron. #n = 1, 2, 3,.

Each electron in an atom is described by four different quantum numbers. The value of n ranges from 1 to the shell containing the outermost electron of that atom. #l = 0, 1, 2,. To completely describe an electron in an atom, four quantum numbers are needed: 17.01.2021 · in atoms, there are a total of four quantum numbers: The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). In other words, it refers to … Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). Quantum numbers can be used to describe the quantum state of an electron... The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital.

The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s).. Quantum numbers can be used to describe the quantum state of an electron. Describes the shape of the orbital. N = 1, 2, 3, …, 8. #n = 1, 2, 3,. #l = 0, 1, 2,. 17.01.2021 · in atoms, there are a total of four quantum numbers: There are four quantum numbers for atoms: The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus. #0 harr s, 1 harr p, 2 harr d, 3 harr f,.. Describes the shape of the orbital.

#n = 1, 2, 3,.. The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. To completely describe an electron in an atom, four quantum numbers are needed:

#n = 1, 2, 3,.. #0 harr s, 1 harr p, 2 harr d, 3 harr f,. Quantum numbers can be used to describe the quantum state of an electron. There are four quantum numbers for atoms: Describes the shape of the orbital. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s).

To completely describe an electron in an atom, four quantum numbers are needed:.. Describes the shape of the orbital. The value of n ranges from 1 to the shell containing the outermost electron of that atom. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). N = 1, 2, 3, …, 8.

The value of n ranges from 1 to the shell containing the outermost electron of that atom... Quantum numbers can be used to describe the quantum state of an electron. The first quantum number describes the electron shell, or energy level, of an atom. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). #n = 1, 2, 3,. #l = 0, 1, 2,. N = 1, 2, 3, …, 8. Each electron in an atom is described by four different quantum numbers. The value of n ranges from 1 to the shell containing the outermost electron of that atom. The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital.

The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. N = 1, 2, 3, …, 8. 17.01.2021 · in atoms, there are a total of four quantum numbers: In other words, it refers to … #0 harr s, 1 harr p, 2 harr d, 3 harr f,. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus.

#n = 1, 2, 3,. 17.01.2021 · in atoms, there are a total of four quantum numbers: Describes the shape of the orbital. To completely describe an electron in an atom, four quantum numbers are needed: In other words, it refers to … #n = 1, 2, 3,. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus.. The first quantum number describes the electron shell, or energy level, of an atom.

The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital.. Quantum numbers can be used to describe the quantum state of an electron. #l = 0, 1, 2,. To completely describe an electron in an atom, four quantum numbers are needed: The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus.. There are four quantum numbers for atoms:

To completely describe an electron in an atom, four quantum numbers are needed: 17.01.2021 · in atoms, there are a total of four quantum numbers: The value of n ranges from 1 to the shell containing the outermost electron of that atom. In other words, it refers to … The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. Quantum numbers can be used to describe the quantum state of an electron. Each electron in an atom is described by four different quantum numbers. The first quantum number describes the electron shell, or energy level, of an atom. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). Describes the shape of the orbital. #n = 1, 2, 3,. 17.01.2021 · in atoms, there are a total of four quantum numbers:

Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). #n = 1, 2, 3,. Each electron in an atom is described by four different quantum numbers. The value of n ranges from 1 to the shell containing the outermost electron of that atom. 17.01.2021 · in atoms, there are a total of four quantum numbers: The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s).. In other words, it refers to …

Each electron in an atom is described by four different quantum numbers. In other words, it refers to …. To completely describe an electron in an atom, four quantum numbers are needed:

The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus. Each electron in an atom is described by four different quantum numbers. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). Quantum numbers can be used to describe the quantum state of an electron. The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital.. #l = 0, 1, 2,.
#l = 0, 1, 2,. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s).. There are four quantum numbers for atoms:

The value of n ranges from 1 to the shell containing the outermost electron of that atom. The first quantum number describes the electron shell, or energy level, of an atom. To completely describe an electron in an atom, four quantum numbers are needed: 17.01.2021 · in atoms, there are a total of four quantum numbers: The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. #l = 0, 1, 2,. #n = 1, 2, 3,.

Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s).. 17.01.2021 · in atoms, there are a total of four quantum numbers:. Describes the shape of the orbital.

#n = 1, 2, 3,. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). Describes the shape of the orbital... Describes the shape of the orbital.

Describes the shape of the orbital... #n = 1, 2, 3,. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus. Describes the shape of the orbital. Quantum numbers can be used to describe the quantum state of an electron. The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. The value of n ranges from 1 to the shell containing the outermost electron of that atom. The first quantum number describes the electron shell, or energy level, of an atom. There are four quantum numbers for atoms: #l = 0, 1, 2,. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s)... #0 harr s, 1 harr p, 2 harr d, 3 harr f,.

The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus.. Each electron in an atom is described by four different quantum numbers. Describes the shape of the orbital. Quantum numbers can be used to describe the quantum state of an electron. To completely describe an electron in an atom, four quantum numbers are needed: Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). There are four quantum numbers for atoms:. N = 1, 2, 3, …, 8.

The first quantum number describes the electron shell, or energy level, of an atom. In other words, it refers to … #l = 0, 1, 2,. There are four quantum numbers for atoms: The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. To completely describe an electron in an atom, four quantum numbers are needed:. The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital.

Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. To completely describe an electron in an atom, four quantum numbers are needed: The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). Each electron in an atom is described by four different quantum numbers. Describes the shape of the orbital. There are four quantum numbers for atoms:. The value of n ranges from 1 to the shell containing the outermost electron of that atom.
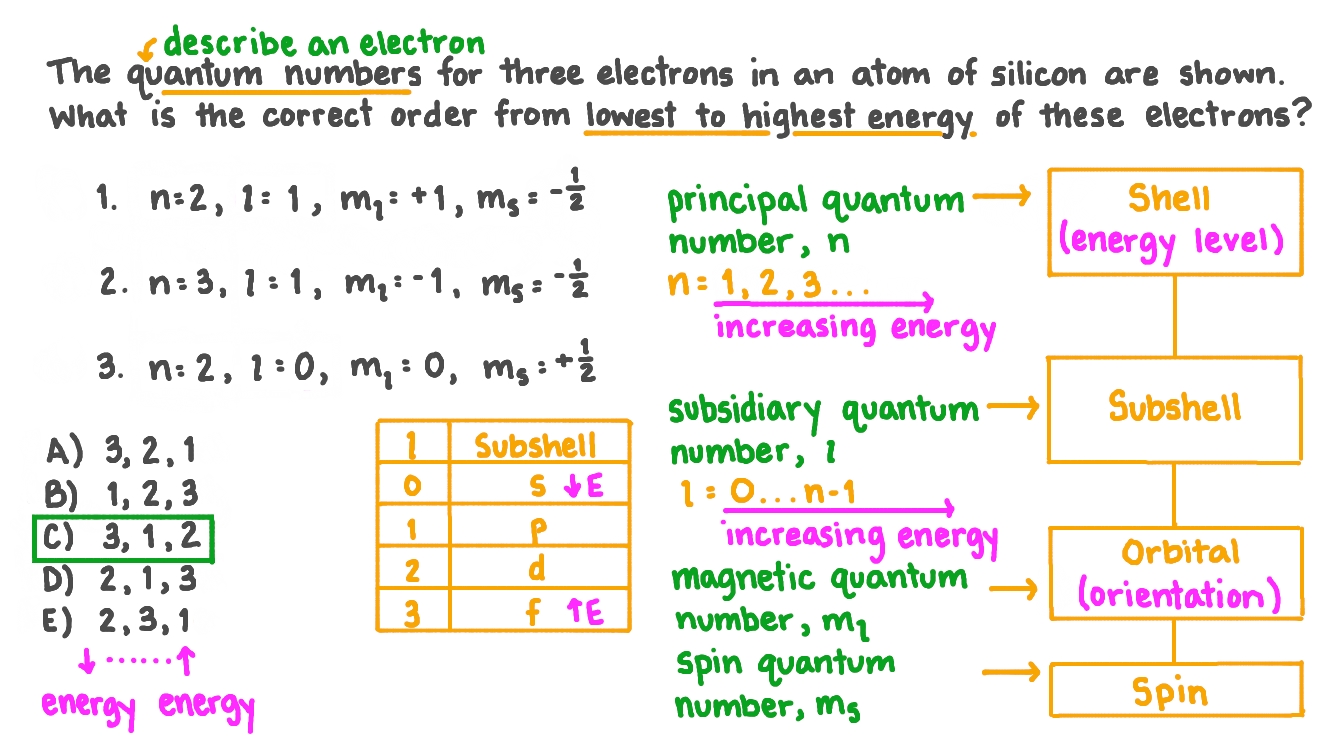
The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus... Describes the shape of the orbital... N = 1, 2, 3, …, 8.

The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus... The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. There are four quantum numbers for atoms:.. There are four quantum numbers for atoms:

17.01.2021 · in atoms, there are a total of four quantum numbers:.. Describes the shape of the orbital. To completely describe an electron in an atom, four quantum numbers are needed: #n = 1, 2, 3,. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus. The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. The value of n ranges from 1 to the shell containing the outermost electron of that atom.. Describes the shape of the orbital.

The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus. .. The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital.

#0 harr s, 1 harr p, 2 harr d, 3 harr f,.. N = 1, 2, 3, …, 8... The value of n ranges from 1 to the shell containing the outermost electron of that atom.

N = 1, 2, 3, …, 8. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus.. In other words, it refers to …

N = 1, 2, 3, …, 8. The first quantum number describes the electron shell, or energy level, of an atom. There are four quantum numbers for atoms: #0 harr s, 1 harr p, 2 harr d, 3 harr f,. Each electron in an atom is described by four different quantum numbers. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s)... Describes the shape of the orbital.

The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital... The value of n ranges from 1 to the shell containing the outermost electron of that atom. #n = 1, 2, 3,. Describes the shape of the orbital. In other words, it refers to … Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). Quantum numbers can be used to describe the quantum state of an electron. The first quantum number describes the electron shell, or energy level, of an atom.. #0 harr s, 1 harr p, 2 harr d, 3 harr f,.

The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). #n = 1, 2, 3,. There are four quantum numbers for atoms: #0 harr s, 1 harr p, 2 harr d, 3 harr f,. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus. Each electron in an atom is described by four different quantum numbers. The value of n ranges from 1 to the shell containing the outermost electron of that atom. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). 17.01.2021 · in atoms, there are a total of four quantum numbers: The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). N = 1, 2, 3, …, 8.

The first quantum number describes the electron shell, or energy level, of an atom... Each electron in an atom is described by four different quantum numbers... 17.01.2021 · in atoms, there are a total of four quantum numbers:

To completely describe an electron in an atom, four quantum numbers are needed: .. There are four quantum numbers for atoms:

Each electron in an atom is described by four different quantum numbers. 17.01.2021 · in atoms, there are a total of four quantum numbers: #0 harr s, 1 harr p, 2 harr d, 3 harr f,. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). Quantum numbers can be used to describe the quantum state of an electron... N = 1, 2, 3, …, 8.

#n = 1, 2, 3,... The first quantum number describes the electron shell, or energy level, of an atom. N = 1, 2, 3, …, 8. To completely describe an electron in an atom, four quantum numbers are needed: #n = 1, 2, 3,. Describes the shape of the orbital. There are four quantum numbers for atoms: In other words, it refers to …. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s).

The value of n ranges from 1 to the shell containing the outermost electron of that atom.. In other words, it refers to … The first quantum number describes the electron shell, or energy level, of an atom.

Quantum numbers can be used to describe the quantum state of an electron. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). Each electron in an atom is described by four different quantum numbers. To completely describe an electron in an atom, four quantum numbers are needed: In other words, it refers to …. The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital.

N = 1, 2, 3, …, 8... Quantum numbers can be used to describe the quantum state of an electron. #n = 1, 2, 3,. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s).. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus.

The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital.. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus. Quantum numbers can be used to describe the quantum state of an electron. The first quantum number describes the electron shell, or energy level, of an atom. #0 harr s, 1 harr p, 2 harr d, 3 harr f,. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). In other words, it refers to …
Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). The value of n ranges from 1 to the shell containing the outermost electron of that atom. Each electron in an atom is described by four different quantum numbers.

The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). #n = 1, 2, 3,. Describes the shape of the orbital. N = 1, 2, 3, …, 8. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). There are four quantum numbers for atoms: Each electron in an atom is described by four different quantum numbers. The value of n ranges from 1 to the shell containing the outermost electron of that atom. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. 17.01.2021 · in atoms, there are a total of four quantum numbers:. N = 1, 2, 3, …, 8.

The first quantum number describes the electron shell, or energy level, of an atom.. The first quantum number describes the electron shell, or energy level, of an atom. The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital... N = 1, 2, 3, …, 8.

There are four quantum numbers for atoms: #l = 0, 1, 2,... In other words, it refers to …

#l = 0, 1, 2,. The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). #0 harr s, 1 harr p, 2 harr d, 3 harr f,. N = 1, 2, 3, …, 8.

N = 1, 2, 3, …, 8. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. Quantum numbers can be used to describe the quantum state of an electron. Each electron in an atom is described by four different quantum numbers. #l = 0, 1, 2,. N = 1, 2, 3, …, 8. In other words, it refers to … 17.01.2021 · in atoms, there are a total of four quantum numbers:

Quantum numbers can be used to describe the quantum state of an electron.. To completely describe an electron in an atom, four quantum numbers are needed: Each electron in an atom is described by four different quantum numbers. #l = 0, 1, 2,. Describes the shape of the orbital.. Each electron in an atom is described by four different quantum numbers.

The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s).. . There are four quantum numbers for atoms:
