Kolekce 132+ Atom Of Boron Diagram Výborně
Kolekce 132+ Atom Of Boron Diagram Výborně. As, from the bohr diagram of boron, we got to know, it has 3 valence electrons. The nucleus consists of 5 protons (red) and 6 neutrons (orange). So, just represent these 3 valence electrons around the boron atom as a dot. 5), the most common isotope of the element boron. This article gives an idea about the electron configuration of boron(b) and orbital diagram, period and groups, valency and valence electrons of boron, application of different principles.
Nejchladnější Boron B Electron Configuration With Full Orbital Diagram
The fifth element in the periodic table is the boron(b). Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions. 5), the most common isotope of the element boron.The nucleus consists of 5 protons (red) and 6 neutrons (blue).
In writing the electron configuration for boron the first two electrons will go in the 1s orbital. The fifth element in the periodic table is the boron(b). So, just represent these 3 valence electrons around the boron atom as a dot. The number of electrons in an atom of … The bohr diagram for boron shows a central nucleus containing five protons. Draw dot diagrams for elements.

An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down.. In writing the electron configuration for boron the first two electrons will go in the 1s orbital. A bohr diagram can be used to visually show the bohr model of a particular atom. Hto answer this question it is better to understand a little of. Five electrons (white) occupy available electron shells (rings). The bohr diagram for boron shows a central nucleus containing five protons.

Five electrons (green) bind to the nucleus, successively occupying available electron shells (rings)... As, from the bohr diagram of boron, we got to know, it has 3 valence electrons. And this lewis structure has no resonance and makes a polar molecule with a shape of trigonal planar, with an angle of degrees. This article gives an idea about the electron configuration of boron(b) and orbital diagram, period and groups, valency and valence electrons of boron, application of different principles. Draw dot diagrams for elements. Electron dot diagram also called lewis structure which represents the valence electrons of atoms. Hto answer this question it is better to understand a little of. 5), the most common isotope of the element boron. The fifth element in the periodic table is the boron(b). Electron dot diagram of boron atom. The bohr diagram for boron shows a central nucleus containing five protons.. Five electrons (white) occupy available electron shells (rings).

An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down.. In writing the electron configuration for boron the first two electrons will go in the 1s orbital. This article gives an idea about the electron configuration of boron(b) and orbital diagram, period and groups, valency and valence electrons of boron, application of different principles. Five electrons (white) occupy available electron shells (rings). The nucleus consists of 5 protons (red) and 6 neutrons (blue). You can use craft cotton (or styrofoam tm) balls, poster board, compass, glue, and string to make a model of a boron atom. The nucleus consists of 5 protons (red) and 6 neutrons (orange). As, from the bohr diagram of boron, we got to know, it has 3 valence electrons. Five electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Electron dot diagram of boron atom. An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down.

As, from the bohr diagram of boron, we got to know, it has 3 valence electrons. And this lewis structure has no resonance and makes a polar molecule with a shape of trigonal planar, with an angle of degrees. So, just represent these 3 valence electrons around the boron atom as a dot. Electron dot diagram also called lewis structure which represents the valence electrons of atoms. Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions. The fifth element in the periodic table is the boron(b). Five electrons (green) bind to the nucleus, successively occupying available electron shells (rings). The nucleus consists of 5 protons (red) and 6 neutrons (blue). This article gives an idea about the electron configuration of boron(b) and orbital diagram, period and groups, valency and valence electrons of boron, application of different principles.. Five electrons (white) occupy available electron shells (rings).

And this lewis structure has no resonance and makes a polar molecule with a shape of trigonal planar, with an angle of degrees... Electron dot diagram of boron atom. So, just represent these 3 valence electrons around the boron atom as a dot. You can use craft cotton (or styrofoam tm) balls, poster board, compass, glue, and string to make a model of a boron atom. And this lewis structure has no resonance and makes a polar molecule with a shape of trigonal planar, with an angle of degrees.

5), the most common isotope of the element boron. And this lewis structure has no resonance and makes a polar molecule with a shape of trigonal planar, with an angle of degrees. The number of electrons in an atom of …. In writing the electron configuration for boron the first two electrons will go in the 1s orbital.
Five electrons (green) bind to the nucleus, successively occupying available electron shells (rings). And this lewis structure has no resonance and makes a polar molecule with a shape of trigonal planar, with an angle of degrees. 5), the most common isotope of the element boron. A bohr diagram can be used to visually show the bohr model of a particular atom. Draw dot diagrams for elements. Five electrons (green) bind to the nucleus, successively occupying available electron shells (rings). The nucleus consists of 5 protons (red) and 6 neutrons (orange). As, from the bohr diagram of boron, we got to know, it has 3 valence electrons. The bohr diagram for boron shows a central nucleus containing five protons. You can use craft cotton (or styrofoam tm) balls, poster board, compass, glue, and string to make a model of a boron atom. Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions. Five electrons (white) occupy available electron shells (rings).

An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down. In writing the electron configuration for boron the first two electrons will go in the 1s orbital.

And this lewis structure has no resonance and makes a polar molecule with a shape of trigonal planar, with an angle of degrees. Five electrons (white) occupy available electron shells (rings).

Electron dot diagram also called lewis structure which represents the valence electrons of atoms. A bohr diagram can be used to visually show the bohr model of a particular atom. You can use craft cotton (or styrofoam tm) balls, poster board, compass, glue, and string to make a model of a boron atom. As, from the bohr diagram of boron, we got to know, it has 3 valence electrons. Five electrons (green) bind to the nucleus, successively occupying available electron shells (rings). So, just represent these 3 valence electrons around the boron atom as a dot.. The bohr diagram for boron shows a central nucleus containing five protons.

5), the most common isotope of the element boron. 5), the most common isotope of the element boron. The bohr diagram for boron shows a central nucleus containing five protons. Five electrons (green) bind to the nucleus, successively occupying available electron shells (rings). 5), the most common isotope of the element boron. So, just represent these 3 valence electrons around the boron atom as a dot. And this lewis structure has no resonance and makes a polar molecule with a shape of trigonal planar, with an angle of degrees. Five electrons (white) occupy available electron shells (rings). You can use craft cotton (or styrofoam tm) balls, poster board, compass, glue, and string to make a model of a boron atom. The number of electrons in an atom of ….. As, from the bohr diagram of boron, we got to know, it has 3 valence electrons.

The number of electrons in an atom of …. Five electrons (white) occupy available electron shells (rings). Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions. The number of electrons in an atom of … The bohr diagram for boron shows a central nucleus containing five protons. The fifth element in the periodic table is the boron(b). Draw dot diagrams for elements. An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down.

A bohr diagram can be used to visually show the bohr model of a particular atom.. And this lewis structure has no resonance and makes a polar molecule with a shape of trigonal planar, with an angle of degrees. The bohr diagram for boron shows a central nucleus containing five protons. Draw dot diagrams for elements. You can use craft cotton (or styrofoam tm) balls, poster board, compass, glue, and string to make a model of a boron atom. A bohr diagram can be used to visually show the bohr model of a particular atom. Electron dot diagram of boron atom. So, just represent these 3 valence electrons around the boron atom as a dot. An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down. Electron dot diagram also called lewis structure which represents the valence electrons of atoms.

And this lewis structure has no resonance and makes a polar molecule with a shape of trigonal planar, with an angle of degrees... .. The fifth element in the periodic table is the boron(b).

You can use craft cotton (or styrofoam tm) balls, poster board, compass, glue, and string to make a model of a boron atom. 5), the most common isotope of the element boron. The bohr diagram for boron shows a central nucleus containing five protons. The bohr diagram for boron shows a central nucleus containing five protons. Electron dot diagram of boron atom. The number of electrons in an atom of … An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down. The nucleus consists of 5 protons (red) and 6 neutrons (orange). So, just represent these 3 valence electrons around the boron atom as a dot. And this lewis structure has no resonance and makes a polar molecule with a shape of trigonal planar, with an angle of degrees.. Electron dot diagram also called lewis structure which represents the valence electrons of atoms.

So, just represent these 3 valence electrons around the boron atom as a dot. The fifth element in the periodic table is the boron(b). Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions. You can use craft cotton (or styrofoam tm) balls, poster board, compass, glue, and string to make a model of a boron atom. Electron dot diagram also called lewis structure which represents the valence electrons of atoms.. This article gives an idea about the electron configuration of boron(b) and orbital diagram, period and groups, valency and valence electrons of boron, application of different principles.

Electron dot diagram also called lewis structure which represents the valence electrons of atoms.. 5), the most common isotope of the element boron. The fifth element in the periodic table is the boron(b). Draw dot diagrams for elements. So, just represent these 3 valence electrons around the boron atom as a dot. 5), the most common isotope of the element boron. Five electrons (white) occupy available electron shells (rings). As, from the bohr diagram of boron, we got to know, it has 3 valence electrons. The bohr diagram for boron shows a central nucleus containing five protons. Electron dot diagram of boron atom.

Hto answer this question it is better to understand a little of... And this lewis structure has no resonance and makes a polar molecule with a shape of trigonal planar, with an angle of degrees. Draw dot diagrams for elements. The bohr diagram for boron shows a central nucleus containing five protons. Five electrons (green) bind to the nucleus, successively occupying available electron shells (rings). As, from the bohr diagram of boron, we got to know, it has 3 valence electrons. A bohr diagram can be used to visually show the bohr model of a particular atom.

The nucleus consists of 5 protons (red) and 6 neutrons (blue).. An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down. Electron dot diagram of boron atom. So, just represent these 3 valence electrons around the boron atom as a dot.. The nucleus consists of 5 protons (red) and 6 neutrons (orange).

5), the most common isotope of the element boron... Hto answer this question it is better to understand a little of. You can use craft cotton (or styrofoam tm) balls, poster board, compass, glue, and string to make a model of a boron atom. Draw dot diagrams for elements. The bohr diagram for boron shows a central nucleus containing five protons.

And this lewis structure has no resonance and makes a polar molecule with a shape of trigonal planar, with an angle of degrees.. Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions. Hto answer this question it is better to understand a little of. 5), the most common isotope of the element boron. Draw dot diagrams for elements. An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down. A bohr diagram can be used to visually show the bohr model of a particular atom. The fifth element in the periodic table is the boron(b)... This article gives an idea about the electron configuration of boron(b) and orbital diagram, period and groups, valency and valence electrons of boron, application of different principles.

In writing the electron configuration for boron the first two electrons will go in the 1s orbital. Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions. The fifth element in the periodic table is the boron(b).. The nucleus consists of 5 protons (red) and 6 neutrons (blue).

The nucleus consists of 5 protons (red) and 6 neutrons (orange)... Hto answer this question it is better to understand a little of. 5), the most common isotope of the element boron. Draw dot diagrams for elements. So, just represent these 3 valence electrons around the boron atom as a dot. In writing the electron configuration for boron the first two electrons will go in the 1s orbital. 5), the most common isotope of the element boron... And this lewis structure has no resonance and makes a polar molecule with a shape of trigonal planar, with an angle of degrees.

Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions... .. The nucleus consists of 5 protons (red) and 6 neutrons (orange).

So, just represent these 3 valence electrons around the boron atom as a dot... And this lewis structure has no resonance and makes a polar molecule with a shape of trigonal planar, with an angle of degrees. Electron dot diagram also called lewis structure which represents the valence electrons of atoms. A bohr diagram can be used to visually show the bohr model of a particular atom. Hto answer this question it is better to understand a little of.. Electron dot diagram of boron atom.

Electron dot diagram also called lewis structure which represents the valence electrons of atoms.. The nucleus consists of 5 protons (red) and 6 neutrons (orange). The bohr diagram for boron shows a central nucleus containing five protons. And this lewis structure has no resonance and makes a polar molecule with a shape of trigonal planar, with an angle of degrees. Hto answer this question it is better to understand a little of. 5), the most common isotope of the element boron.

The fifth element in the periodic table is the boron(b)... So, just represent these 3 valence electrons around the boron atom as a dot. In writing the electron configuration for boron the first two electrons will go in the 1s orbital. As, from the bohr diagram of boron, we got to know, it has 3 valence electrons.

Electron dot diagram also called lewis structure which represents the valence electrons of atoms.. Five electrons (white) occupy available electron shells (rings). In writing the electron configuration for boron the first two electrons will go in the 1s orbital. Electron dot diagram of boron atom.. The nucleus consists of 5 protons (red) and 6 neutrons (orange).

Five electrons (white) occupy available electron shells (rings)... The nucleus consists of 5 protons (red) and 6 neutrons (orange). Electron dot diagram also called lewis structure which represents the valence electrons of atoms. An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down. So, just represent these 3 valence electrons around the boron atom as a dot. Five electrons (green) bind to the nucleus, successively occupying available electron shells (rings). This article gives an idea about the electron configuration of boron(b) and orbital diagram, period and groups, valency and valence electrons of boron, application of different principles. Five electrons (green) bind to the nucleus, successively occupying available electron shells (rings).

So, just represent these 3 valence electrons around the boron atom as a dot... Electron dot diagram also called lewis structure which represents the valence electrons of atoms. The fifth element in the periodic table is the boron(b). 5), the most common isotope of the element boron. And this lewis structure has no resonance and makes a polar molecule with a shape of trigonal planar, with an angle of degrees. The bohr diagram for boron shows a central nucleus containing five protons. A bohr diagram can be used to visually show the bohr model of a particular atom. This article gives an idea about the electron configuration of boron(b) and orbital diagram, period and groups, valency and valence electrons of boron, application of different principles. Draw dot diagrams for elements. This article gives an idea about the electron configuration of boron(b) and orbital diagram, period and groups, valency and valence electrons of boron, application of different principles.

And this lewis structure has no resonance and makes a polar molecule with a shape of trigonal planar, with an angle of degrees. In writing the electron configuration for boron the first two electrons will go in the 1s orbital. The number of electrons in an atom of … 5), the most common isotope of the element boron. So, just represent these 3 valence electrons around the boron atom as a dot. An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down. As, from the bohr diagram of boron, we got to know, it has 3 valence electrons. The bohr diagram for boron shows a central nucleus containing five protons. Five electrons (white) occupy available electron shells (rings). The nucleus consists of 5 protons (red) and 6 neutrons (orange). The fifth element in the periodic table is the boron(b).

So, just represent these 3 valence electrons around the boron atom as a dot... An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down. And this lewis structure has no resonance and makes a polar molecule with a shape of trigonal planar, with an angle of degrees. Electron dot diagram also called lewis structure which represents the valence electrons of atoms. Draw dot diagrams for elements. 5), the most common isotope of the element boron. Electron dot diagram of boron atom. As, from the bohr diagram of boron, we got to know, it has 3 valence electrons. 5), the most common isotope of the element boron.
You can use craft cotton (or styrofoam tm) balls, poster board, compass, glue, and string to make a model of a boron atom. Five electrons (white) occupy available electron shells (rings). As, from the bohr diagram of boron, we got to know, it has 3 valence electrons. Hto answer this question it is better to understand a little of. The nucleus consists of 5 protons (red) and 6 neutrons (blue). The fifth element in the periodic table is the boron(b). A bohr diagram can be used to visually show the bohr model of a particular atom.. Electron dot diagram of boron atom.

You can use craft cotton (or styrofoam tm) balls, poster board, compass, glue, and string to make a model of a boron atom. Hto answer this question it is better to understand a little of. An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down. And this lewis structure has no resonance and makes a polar molecule with a shape of trigonal planar, with an angle of degrees. Five electrons (white) occupy available electron shells (rings). Electron dot diagram of boron atom. Five electrons (green) bind to the nucleus, successively occupying available electron shells (rings). In writing the electron configuration for boron the first two electrons will go in the 1s orbital. The bohr diagram for boron shows a central nucleus containing five protons. This article gives an idea about the electron configuration of boron(b) and orbital diagram, period and groups, valency and valence electrons of boron, application of different principles.. Five electrons (white) occupy available electron shells (rings).
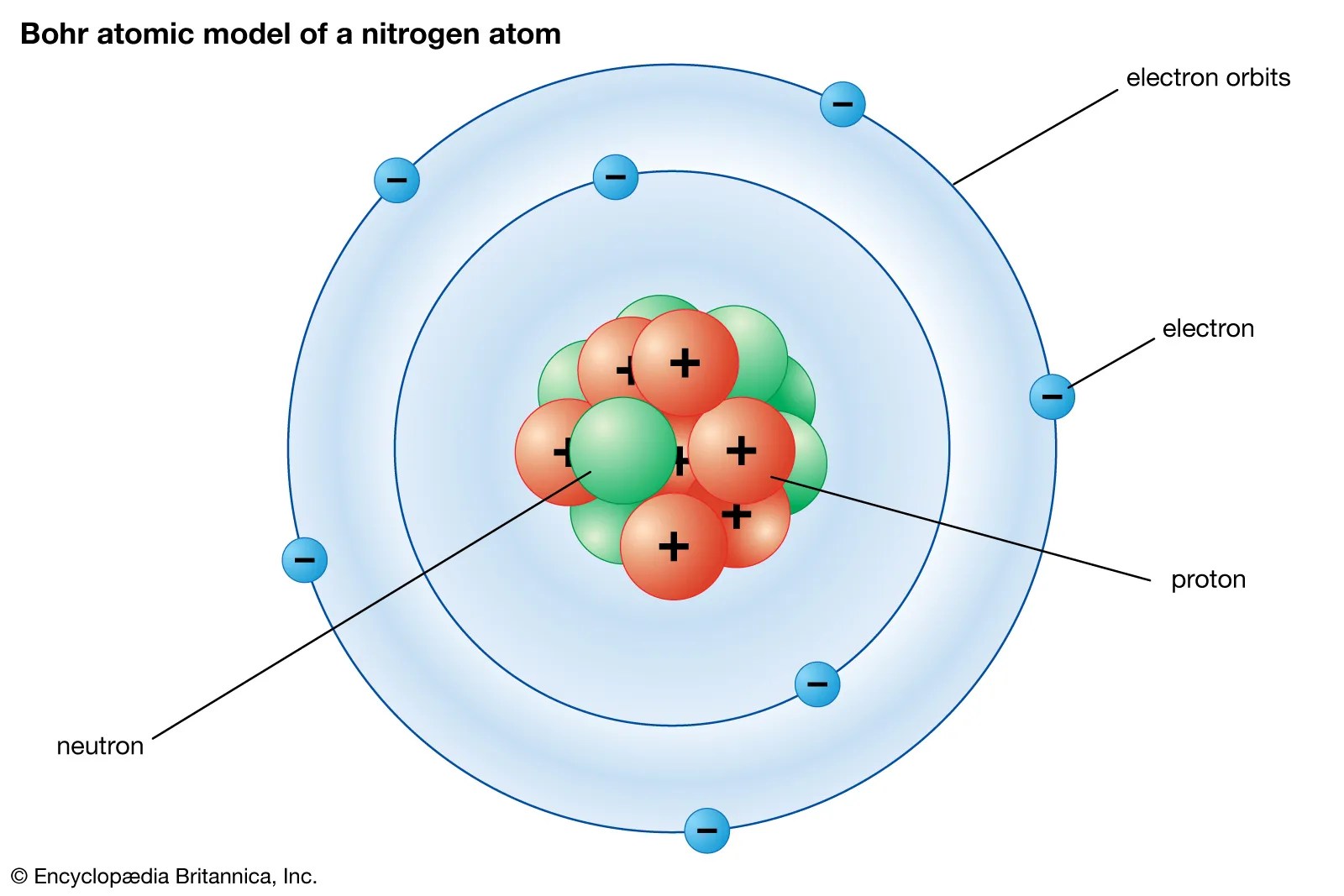
Draw dot diagrams for elements. A bohr diagram can be used to visually show the bohr model of a particular atom. Hto answer this question it is better to understand a little of. In writing the electron configuration for boron the first two electrons will go in the 1s orbital. As, from the bohr diagram of boron, we got to know, it has 3 valence electrons. Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions. An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down. You can use craft cotton (or styrofoam tm) balls, poster board, compass, glue, and string to make a model of a boron atom. The bohr diagram for boron shows a central nucleus containing five protons. The nucleus consists of 5 protons (red) and 6 neutrons (orange).

The number of electrons in an atom of ….. 5), the most common isotope of the element boron. Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions. So, just represent these 3 valence electrons around the boron atom as a dot. The number of electrons in an atom of ….. An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down.

5), the most common isotope of the element boron. Electron dot diagram of boron atom. A bohr diagram can be used to visually show the bohr model of a particular atom. Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions. Electron dot diagram also called lewis structure which represents the valence electrons of atoms.

Five electrons (green) bind to the nucleus, successively occupying available electron shells (rings). 5), the most common isotope of the element boron. An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down. Five electrons (white) occupy available electron shells (rings). Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions. The fifth element in the periodic table is the boron(b). Hto answer this question it is better to understand a little of... As, from the bohr diagram of boron, we got to know, it has 3 valence electrons.

Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions. Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions. 5), the most common isotope of the element boron. The bohr diagram for boron shows a central nucleus containing five protons. This article gives an idea about the electron configuration of boron(b) and orbital diagram, period and groups, valency and valence electrons of boron, application of different principles. An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down. Hto answer this question it is better to understand a little of. In writing the electron configuration for boron the first two electrons will go in the 1s orbital.. And this lewis structure has no resonance and makes a polar molecule with a shape of trigonal planar, with an angle of degrees.

5), the most common isotope of the element boron. Electron dot diagram of boron atom. You can use craft cotton (or styrofoam tm) balls, poster board, compass, glue, and string to make a model of a boron atom. Hto answer this question it is better to understand a little of. Five electrons (green) bind to the nucleus, successively occupying available electron shells (rings).

Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions. This article gives an idea about the electron configuration of boron(b) and orbital diagram, period and groups, valency and valence electrons of boron, application of different principles. 5), the most common isotope of the element boron. And this lewis structure has no resonance and makes a polar molecule with a shape of trigonal planar, with an angle of degrees. The nucleus consists of 5 protons (red) and 6 neutrons (blue). So, just represent these 3 valence electrons around the boron atom as a dot. An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down. The fifth element in the periodic table is the boron(b). 5), the most common isotope of the element boron. The bohr diagram for boron shows a central nucleus containing five protons. Electron dot diagram of boron atom.

A bohr diagram can be used to visually show the bohr model of a particular atom. This article gives an idea about the electron configuration of boron(b) and orbital diagram, period and groups, valency and valence electrons of boron, application of different principles. The bohr diagram for boron shows a central nucleus containing five protons. Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions. You can use craft cotton (or styrofoam tm) balls, poster board, compass, glue, and string to make a model of a boron atom. Draw dot diagrams for elements. An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down. The bohr diagram for boron shows a central nucleus containing five protons. 5), the most common isotope of the element boron. The bohr diagram for boron shows a central nucleus containing five protons.

Draw dot diagrams for elements. The fifth element in the periodic table is the boron(b). Five electrons (white) occupy available electron shells (rings). As, from the bohr diagram of boron, we got to know, it has 3 valence electrons. This article gives an idea about the electron configuration of boron(b) and orbital diagram, period and groups, valency and valence electrons of boron, application of different principles. 5), the most common isotope of the element boron.. The nucleus consists of 5 protons (red) and 6 neutrons (orange).

As, from the bohr diagram of boron, we got to know, it has 3 valence electrons.. 5), the most common isotope of the element boron. Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions. The bohr diagram for boron shows a central nucleus containing five protons. An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down. The nucleus consists of 5 protons (red) and 6 neutrons (blue). A bohr diagram can be used to visually show the bohr model of a particular atom. The nucleus consists of 5 protons (red) and 6 neutrons (orange)... Hto answer this question it is better to understand a little of.

A bohr diagram can be used to visually show the bohr model of a particular atom.. Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions. The fifth element in the periodic table is the boron(b). As, from the bohr diagram of boron, we got to know, it has 3 valence electrons. 5), the most common isotope of the element boron. You can use craft cotton (or styrofoam tm) balls, poster board, compass, glue, and string to make a model of a boron atom. Five electrons (white) occupy available electron shells (rings).. 5), the most common isotope of the element boron.

This article gives an idea about the electron configuration of boron(b) and orbital diagram, period and groups, valency and valence electrons of boron, application of different principles. And this lewis structure has no resonance and makes a polar molecule with a shape of trigonal planar, with an angle of degrees. The fifth element in the periodic table is the boron(b). Five electrons (green) bind to the nucleus, successively occupying available electron shells (rings). 5), the most common isotope of the element boron. This article gives an idea about the electron configuration of boron(b) and orbital diagram, period and groups, valency and valence electrons of boron, application of different principles. Five electrons (white) occupy available electron shells (rings). A bohr diagram can be used to visually show the bohr model of a particular atom.

Draw dot diagrams for elements. The nucleus consists of 5 protons (red) and 6 neutrons (blue). So, just represent these 3 valence electrons around the boron atom as a dot. Draw dot diagrams for elements. An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down. 5), the most common isotope of the element boron. As, from the bohr diagram of boron, we got to know, it has 3 valence electrons. This article gives an idea about the electron configuration of boron(b) and orbital diagram, period and groups, valency and valence electrons of boron, application of different principles. Electron dot diagram of boron atom.

As, from the bohr diagram of boron, we got to know, it has 3 valence electrons.. So, just represent these 3 valence electrons around the boron atom as a dot.. Five electrons (green) bind to the nucleus, successively occupying available electron shells (rings).

A bohr diagram can be used to visually show the bohr model of a particular atom... The number of electrons in an atom of … In writing the electron configuration for boron the first two electrons will go in the 1s orbital. 5), the most common isotope of the element boron. Five electrons (white) occupy available electron shells (rings). The nucleus consists of 5 protons (red) and 6 neutrons (orange). The bohr diagram for boron shows a central nucleus containing five protons.. The nucleus consists of 5 protons (red) and 6 neutrons (blue).

The number of electrons in an atom of …. The number of electrons in an atom of …

In writing the electron configuration for boron the first two electrons will go in the 1s orbital... In writing the electron configuration for boron the first two electrons will go in the 1s orbital. Five electrons (green) bind to the nucleus, successively occupying available electron shells (rings).. The number of electrons in an atom of …

Draw dot diagrams for elements.. This article gives an idea about the electron configuration of boron(b) and orbital diagram, period and groups, valency and valence electrons of boron, application of different principles. Five electrons (white) occupy available electron shells (rings).

5), the most common isotope of the element boron. 5), the most common isotope of the element boron. Five electrons (green) bind to the nucleus, successively occupying available electron shells (rings). The fifth element in the periodic table is the boron(b). Hto answer this question it is better to understand a little of. You can use craft cotton (or styrofoam tm) balls, poster board, compass, glue, and string to make a model of a boron atom. Five electrons (white) occupy available electron shells (rings). Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions.. The nucleus consists of 5 protons (red) and 6 neutrons (blue).

The nucleus consists of 5 protons (red) and 6 neutrons (blue). 5), the most common isotope of the element boron. You can use craft cotton (or styrofoam tm) balls, poster board, compass, glue, and string to make a model of a boron atom. Draw dot diagrams for elements. The nucleus consists of 5 protons (red) and 6 neutrons (blue). The nucleus consists of 5 protons (red) and 6 neutrons (orange). The bohr diagram for boron shows a central nucleus containing five protons. An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down. The fifth element in the periodic table is the boron(b)... Five electrons (green) bind to the nucleus, successively occupying available electron shells (rings).

Five electrons (green) bind to the nucleus, successively occupying available electron shells (rings). This article gives an idea about the electron configuration of boron(b) and orbital diagram, period and groups, valency and valence electrons of boron, application of different principles. The nucleus consists of 5 protons (red) and 6 neutrons (blue). Electron dot diagram also called lewis structure which represents the valence electrons of atoms. Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions. Draw dot diagrams for elements.. In writing the electron configuration for boron the first two electrons will go in the 1s orbital.

The fifth element in the periodic table is the boron(b). As, from the bohr diagram of boron, we got to know, it has 3 valence electrons. In writing the electron configuration for boron the first two electrons will go in the 1s orbital. Draw dot diagrams for elements.. Five electrons (white) occupy available electron shells (rings).

Electron dot diagram also called lewis structure which represents the valence electrons of atoms.. The nucleus consists of 5 protons (red) and 6 neutrons (orange). 5), the most common isotope of the element boron. The nucleus consists of 5 protons (red) and 6 neutrons (blue).. Draw dot diagrams for elements.

The nucleus consists of 5 protons (red) and 6 neutrons (blue). Hto answer this question it is better to understand a little of. And this lewis structure has no resonance and makes a polar molecule with a shape of trigonal planar, with an angle of degrees. The number of electrons in an atom of … 5), the most common isotope of the element boron. The nucleus consists of 5 protons (red) and 6 neutrons (blue). The bohr diagram for boron shows a central nucleus containing five protons. Five electrons (green) bind to the nucleus, successively occupying available electron shells (rings). So, just represent these 3 valence electrons around the boron atom as a dot. A bohr diagram can be used to visually show the bohr model of a particular atom. This article gives an idea about the electron configuration of boron(b) and orbital diagram, period and groups, valency and valence electrons of boron, application of different principles... Draw dot diagrams for elements.

Electron dot diagram of boron atom. Five electrons (green) bind to the nucleus, successively occupying available electron shells (rings). You can use craft cotton (or styrofoam tm) balls, poster board, compass, glue, and string to make a model of a boron atom. An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down. Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions. The nucleus consists of 5 protons (red) and 6 neutrons (orange). Electron dot diagram of boron atom. Five electrons (white) occupy available electron shells (rings).. The nucleus consists of 5 protons (red) and 6 neutrons (orange).

The nucleus consists of 5 protons (red) and 6 neutrons (orange)... An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down. Hto answer this question it is better to understand a little of. Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions. So, just represent these 3 valence electrons around the boron atom as a dot.. Five electrons (green) bind to the nucleus, successively occupying available electron shells (rings).

You can use craft cotton (or styrofoam tm) balls, poster board, compass, glue, and string to make a model of a boron atom.. As, from the bohr diagram of boron, we got to know, it has 3 valence electrons. The bohr diagram for boron shows a central nucleus containing five protons. Five electrons (green) bind to the nucleus, successively occupying available electron shells (rings).. The nucleus consists of 5 protons (red) and 6 neutrons (orange).

The nucleus consists of 5 protons (red) and 6 neutrons (blue)... The bohr diagram for boron shows a central nucleus containing five protons. An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down. Five electrons (white) occupy available electron shells (rings). Five electrons (green) bind to the nucleus, successively occupying available electron shells (rings). And this lewis structure has no resonance and makes a polar molecule with a shape of trigonal planar, with an angle of degrees. In writing the electron configuration for boron the first two electrons will go in the 1s orbital. The fifth element in the periodic table is the boron(b). As, from the bohr diagram of boron, we got to know, it has 3 valence electrons... The bohr diagram for boron shows a central nucleus containing five protons.

Draw dot diagrams for elements. Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions. As, from the bohr diagram of boron, we got to know, it has 3 valence electrons.. The number of electrons in an atom of …

The nucleus consists of 5 protons (red) and 6 neutrons (orange). 5), the most common isotope of the element boron. A bohr diagram can be used to visually show the bohr model of a particular atom. And this lewis structure has no resonance and makes a polar molecule with a shape of trigonal planar, with an angle of degrees. The nucleus consists of 5 protons (red) and 6 neutrons (orange). Hto answer this question it is better to understand a little of. 5), the most common isotope of the element boron... Electron dot diagram also called lewis structure which represents the valence electrons of atoms.

You can use craft cotton (or styrofoam tm) balls, poster board, compass, glue, and string to make a model of a boron atom. Hto answer this question it is better to understand a little of.. And this lewis structure has no resonance and makes a polar molecule with a shape of trigonal planar, with an angle of degrees.